Medical Equipment
- Home
- Aerospace
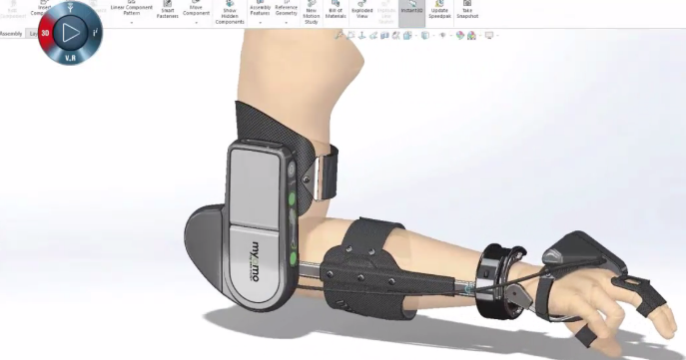
Panshul Technologies is an authorized SOLIDWORKS Reseller for Medical Equipment Design in Mumbai, Navi Mumbai, Thane, Nashik | Book a Free Demo & Get the Best Price on SOLIDWORKS

Medical Equipment SOLIDWORKS
SOLIDWORKS supports the medical device sector with powerful design and simulation tools that accelerate innovation while maintaining compliance. From concept to production, teams can model intricate assemblies, simulate usage scenarios, and generate documentation for regulatory submissions. This reduces development cycles and helps bring safer, more reliable medical equipment to market faster, with fewer design errors and enhanced collaboration between teams.
To know more
ABOUT THE PRODUCT book a free demo
How SOLIDWORKS helped these industries
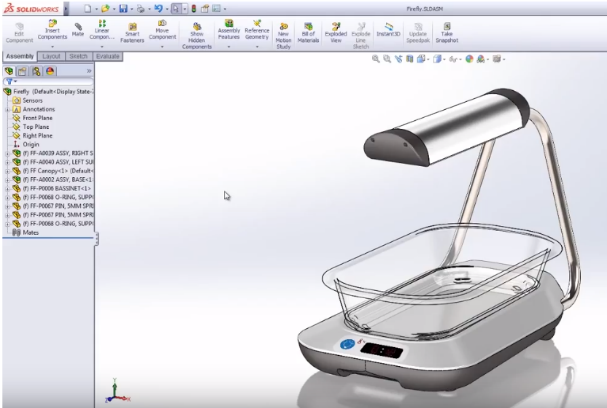
Accelerated Device Innovation
SOLIDWORKS helped companies rapidly prototype and test devices like pacemakers, surgical tools, and implants using intuitive 3D modeling.
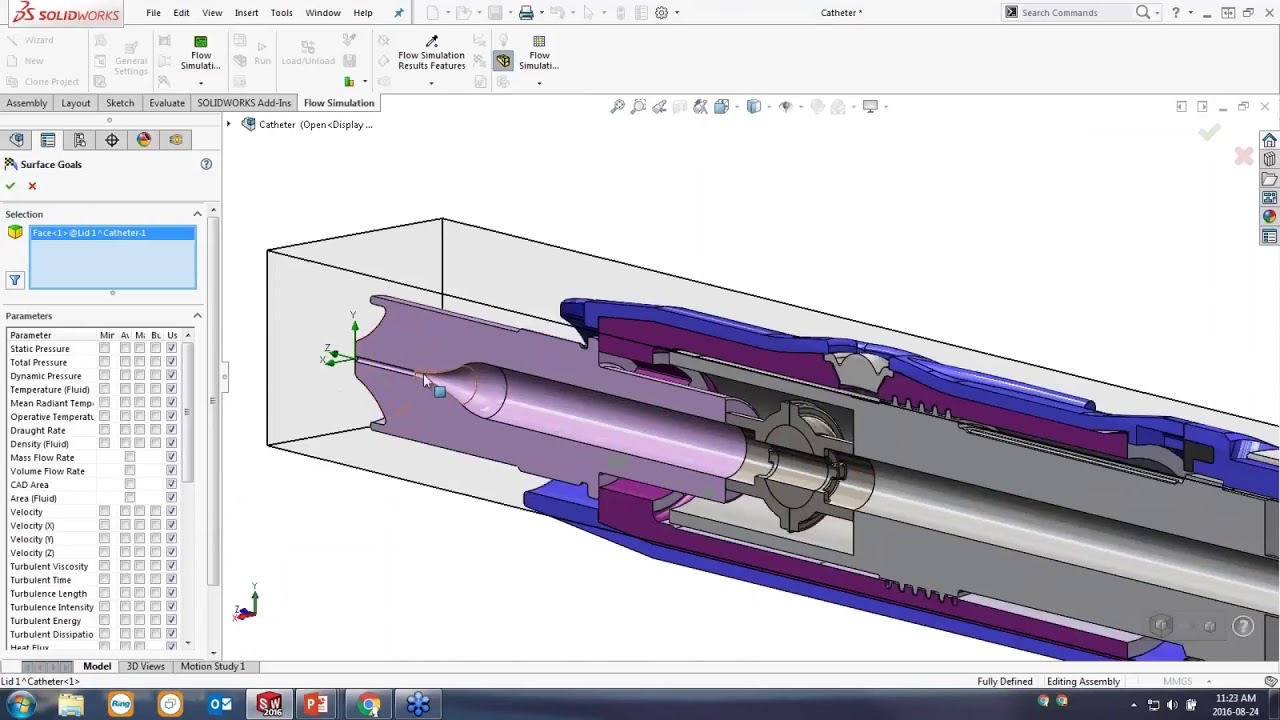
Compliance-Ready Documentation
Teams generated 2D drawings, exploded views, and inspection reports for FDA or CE mark submissions.
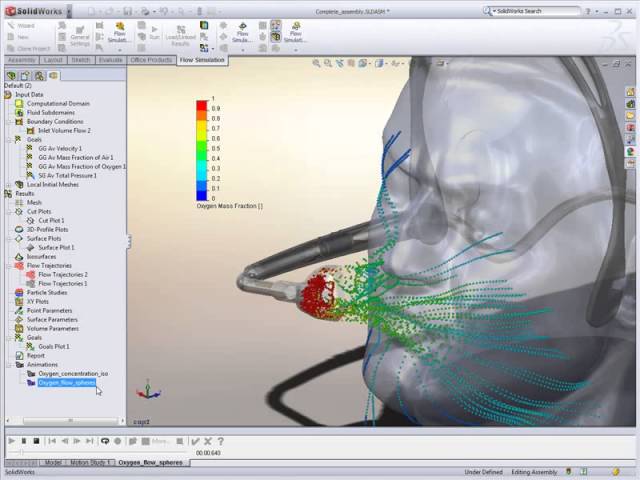
Simulation for Real-World Scenarios
Engineers used SOLIDWORKS Simulation to test stress, strain, and impact on components that interact with human tissue or medical environments.
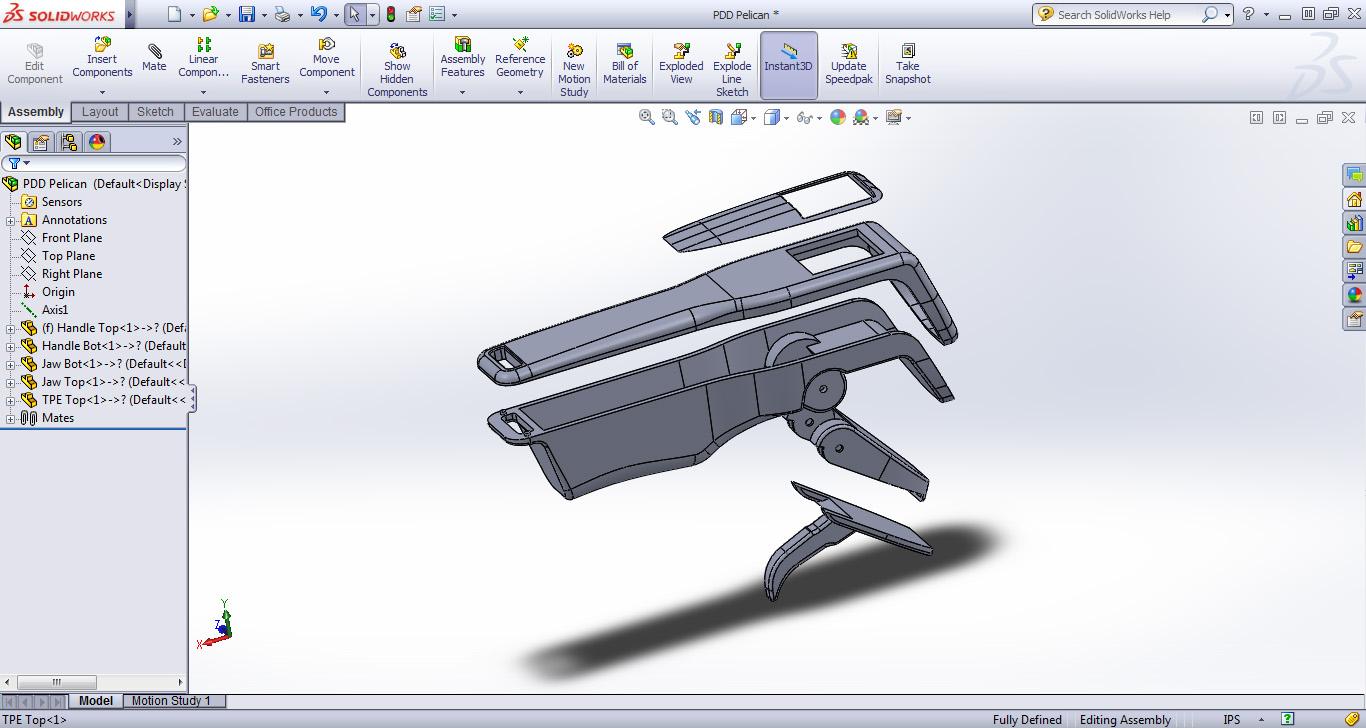
Compact and Ergonomic Design
Parametric tools allowed designers to create space-efficient, user-friendly medical equipment for both hospital and home use.
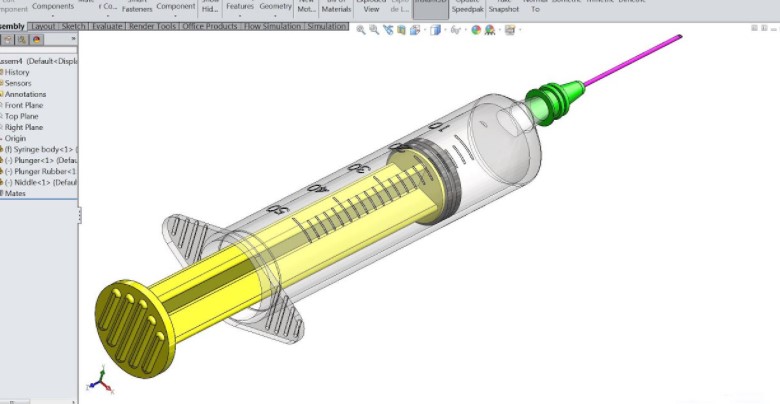
Improved Sterilization Planning
Design validation ensured that all components were compatible with sterilization techniques like autoclaving or UV treatment.
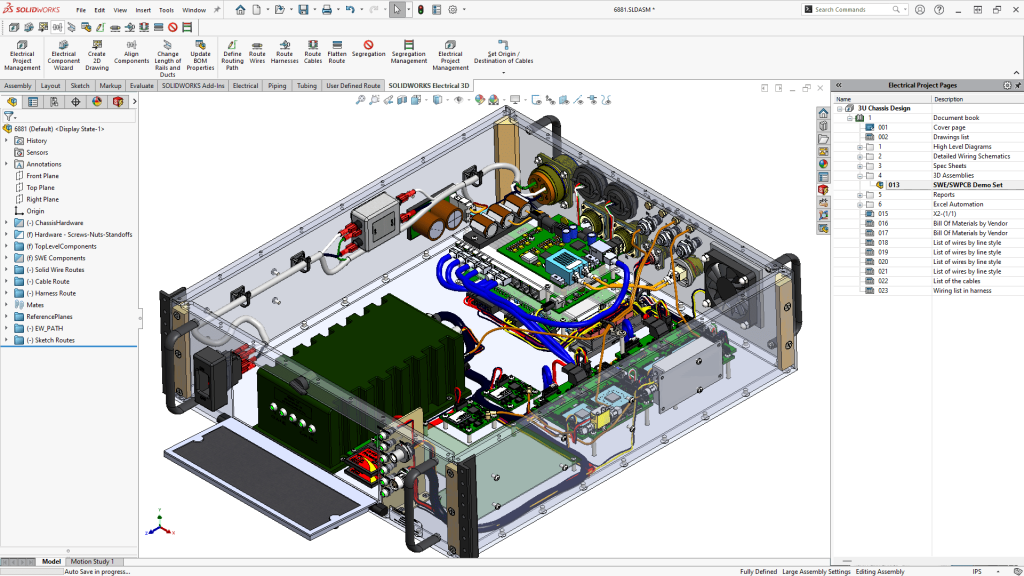
Integrated Electromechanical Design
With SOLIDWORKS Electrical, companies streamlined development of mechatronic systems used in diagnostic and monitoring devices.
Related Products

SOLIDWORKS Premium
Benefits:
- Simulate real-world conditions like load, pressure, and vibration.
- Validate biocompatible material performance and reliability.
- Reduce need for physical testing and improve safety.

SOLIDWORKS Simulation
Benefits:
- Perform static, dynamic, thermal, and fatigue analysis on medical components.
- Ensure product safety, strength, and durability under repeated use.
- Reduce need for physical testing, speeding up certification processes (FDA/CE).

SOLIDWORKS Electrical
Benefits:
- Design circuits for powered medical equipment and sensors.
- Ensure alignment between electrical and mechanical designs.
- Reduce wiring errors in ECGs, ventilators, or lab devices.

SOLIDWORKS MBD (Model-Based Definition)
Benefits:
- Define tolerances, notes, and PMI directly on 3D models.
- Streamline regulatory submissions with fewer 2D drawings.
- Ensure manufacturing precision through clear digital specs.

SOLIDWORKS Inspection
Benefits:
- Create first-article inspection reports with ease.
- Track quality compliance for medical-grade parts.
- Minimize delays in certification or batch production

SOLIDWORKS Composer
Benefits:
- Develop detailed product manuals and usage guides.
- Help surgeons or technicians understand device usage.
- Support training with interactive 3D visuals

3DEXPERIENCE Works
Benefits:
- Centralize collaboration between R&D, QA, and compliance.
- Track approval cycles and version control in real time.
- Accelerate go-to-market for Class I, II, or III devices




